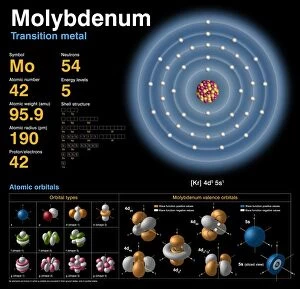
Atomic Weight Collection
Atomic weight is a fundamental concept in chemistry that was first explored by Pierre Dulong, a brilliant French chemist
For sale as Licensed Images
Choose your image, Select your licence and Download the media
Atomic weight is a fundamental concept in chemistry that was first explored by Pierre Dulong, a brilliant French chemist. It refers to the average mass of an atom of an element, taking into account all its naturally occurring isotopes. One fascinating example can be seen in Rutherfordium, a synthetic element named after Ernest Rutherford, the renowned physicist who revolutionized our understanding of atomic structure. With an atomic number of 104 and symbolized as Rf on the periodic table, Rutherfordium's atomic weight is determined by summing up the masses of its protons and neutrons. Speaking of atomic structure, let's delve deeper into Praseodymium - another intriguing element with unique properties. Found within the lanthanide series and denoted by symbol Pr, Praseodymium showcases a distinct arrangement of electrons around its nucleus. However, it is important to note that while elements like Rutherfordium and Praseodymium have their own specific atomic structures contributing to their respective weights, other elements such as Phosphorus also possess intricate arrangements within their atoms. Phosphorus (symbol P) has an electron configuration denoted as C018 / 3696 – this signifies how electrons occupy different energy levels or orbitals. In summary, understanding atomic weight requires exploring various aspects including historical contributions from scientists like Pierre Dulong and investigating the intricate compositions found within elements such as Rutherfordium with its unique atomic structure. Elements like Praseodymium further emphasize how electron configurations play a crucial role in determining overall mass. So next time you encounter these elements on the periodic table or ponder over chemical equations involving them, remember that behind every formula lies a captivating world governed by atomic weights and structures.