Chemisch Collection
"Exploring the Fascinating World of Chemisch: From Fruit-Powered Clocks to Atomic Models and Molecules" Discovering the wonders of chemisch
For sale as Licensed Images
Choose your image, Select your licence and Download the media
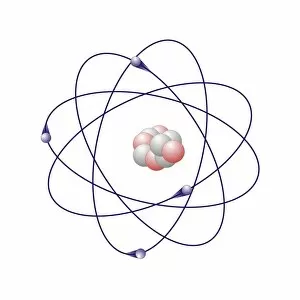
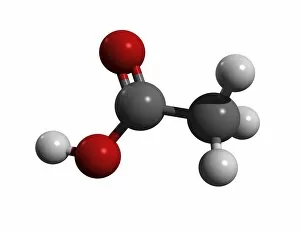
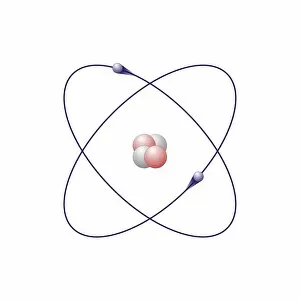
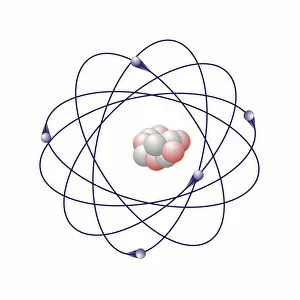
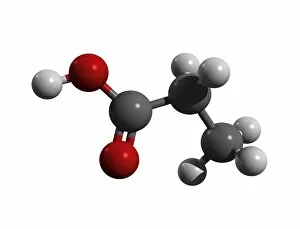
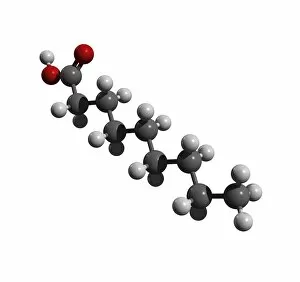
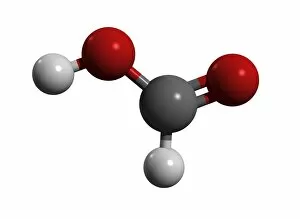
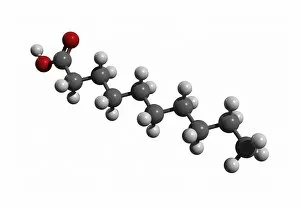
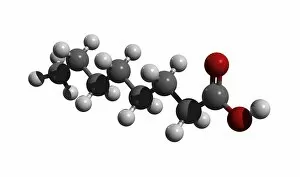
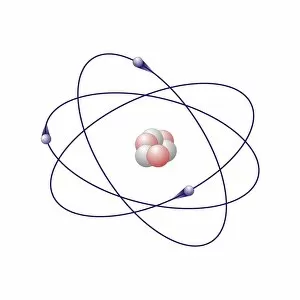
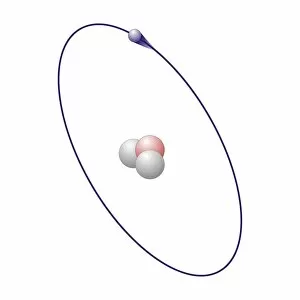
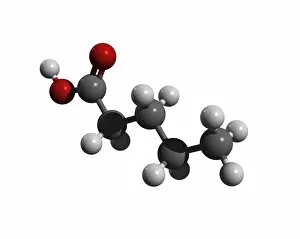
"Exploring the Fascinating World of Chemisch: From Fruit-Powered Clocks to Atomic Models and Molecules" Discovering the wonders of chemisch, we delve into a realm where science meets curiosity. Starting with a fruit-powered clock, this captivating invention showcases the power of chemical reactions harnessed from nature's bountiful fruits. Moving deeper into the atomic level, we encounter Beryllium's intricate atomic model. Its unique structure offers insights into its properties and potential applications in various fields. Similarly, Helium's atomic model unravels its lightness and non-reactive nature that make it an essential element for many scientific endeavors. Zooming in further, our attention is drawn towards molecules like Propanoic acid – a compound found in cheese responsible for its distinct aroma. Acetic acid follows suit, known for its presence in vinegar and contributing to its tangy taste. As we explore more molecules on this chemical journey, Pelargonic acid comes into focus with its role as an important ingredient in cosmetics and fragrances. Formic acid takes center stage next – known for being produced by ants as their defense mechanism but also utilized industrially. The spotlight then shifts to Capric acid; present abundantly in coconut oil and renowned for numerous health benefits. We encounter Caprylic acid once again - another fatty acid found naturally in coconuts with antimicrobial properties. Finally, Butyric Acid wraps up our exploration – recognized by its pungent smell often associated with rancid butter or vomit but also playing vital roles within our digestive system. Chemisch invites us to appreciate the intricacies of chemistry through these diverse hints - from fruit-powered clocks demonstrating energy conversion to exploring atomic models unraveling elements' behavior at microscopic levels alongside fascinating molecules shaping scents, flavors, cosmetics & even human physiology.