Ercker Collection
In the year 1683, Lazarus Ercker took us on a captivating journey through the intricate world of metal production and refinement
For sale as Licensed Images
Choose your image, Select your licence and Download the media
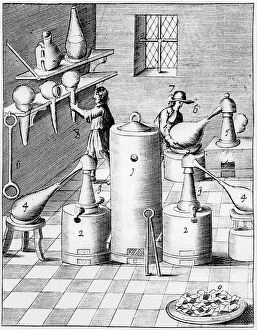
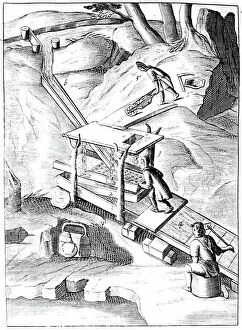
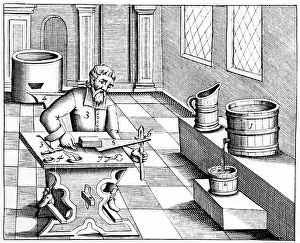
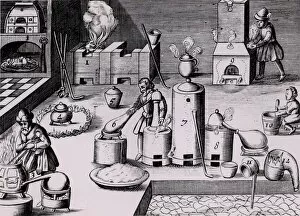
In the year 1683, Lazarus Ercker took us on a captivating journey through the intricate world of metal production and refinement. His meticulous attention to detail is evident in his depictions of various processes that were essential for creating coins and other valuable items. One such process involved the preparation of copper and silver alloys, which served as the foundation for coin production. Ercker's illustrations showcase skilled craftsmen carefully measuring and combining these metals, ensuring their composition was perfect. Another fascinating aspect captured by the crystallization of saltpetre or potassium nitrate. This vital substance was used in various applications, including gunpowder production. Through his artistry, we witness scientists observing its quality with great precision, guaranteeing its effectiveness. It also takes us into a laboratory dedicated to refining gold and silver. The scene reveals an array of sophisticated equipment meticulously arranged to carry out this delicate task, and is here that precious metals are purified to achieve their utmost brilliance before being transformed into exquisite objects. The artist further enlightens us about the production of saltpetre itself - a complex procedure involving careful chemical reactions and precise measurements. These insights shed light on how crucial this compound was during that era. Amongst Ercker's illustrations lies a depiction of distillation - specifically showcasing the process of obtaining nitric acid from raw materials. This technique required skillful manipulation of heat and condensation to extract this powerful substance successfully. An intriguing invention showcased by Ercker is an Athanor or Slow Harry furnace - an ingenious self-feeding contraption designed to maintain a constant temperature over extended periods. Such furnaces played pivotal roles in processes like cementation where protracted heat was necessary for desired outcomes. It also introduces us to another type of furnace used in ore extraction: one requiring prolonged heating known as cementation furnace dating back even earlier than his time period (1580). This method allowed miners to wash ores meticulously, extracting precious gold particles hidden within.