Gases Collection (#4)
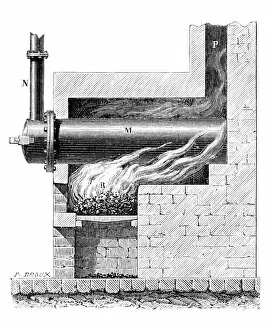
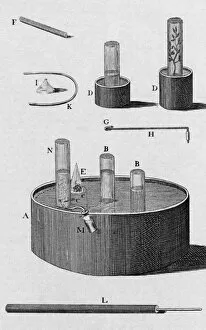
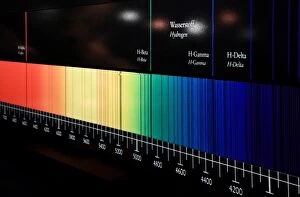
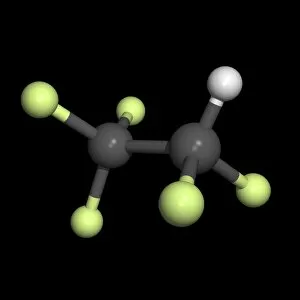
"Gases: Unveiling the Invisible Marvels of our Universe" Humphry Davy's groundbreaking discoveries in gases revolutionized scientific understanding
For sale as Licensed Images
Choose your image, Select your licence and Download the media
"Gases: Unveiling the Invisible Marvels of our Universe" Humphry Davy's groundbreaking discoveries in gases revolutionized scientific understanding. James Clerk Maxwell, the Scottish physicist, contributed to our knowledge of gas behavior with his insightful theories. Water vapor maps of Antarctica in 2004 shed light on the complex dynamics of this essential gas. The intricate artwork depicting a DNA molecule reminds us that even life itself relies on gaseous interactions. Witnessing the Fagradalsfjall volcano eruption at night showcases the raw power and beauty of volcanic gases. Cross-section views of lava eruptions provide a mesmerizing glimpse into the fiery world where molten rock meets gaseous emissions. The Helix Nebula captured by VISTA reveals stunning celestial formations shaped by interstellar gases. The captivating sight of lava meeting the ocean highlights nature's ability to shape coastlines through volatile gas reactions. Svartsengi Geothermal plant fuels Iceland's famous outdoor spa, The Blue Lagoon, using naturally occurring underground gases for relaxation and healing purposes. Sir William Ramsay's Nobel Prize-winning contributions to chemistry include unraveling mysteries surrounding various gases' properties and behaviors. Spanish Civil War: Instructions on how to deal with toxic gases during wartime underscored their destructive potential and urgent need for protection measures. John Dalton's pioneering work as an English chemist and physicist laid the foundation for modern atomic theory, including our understanding of how different elements combine to form compounds. From Earth’s geothermal wonders to cosmic phenomena, from industrial applications to fundamental scientific breakthroughs—gases permeate every aspect of our existence, often hidden but always influential.