Thermodynamics Collection (#2)
Thermodynamics, a fascinating branch of physics, owes its foundations to the brilliant minds of James Clerk Maxwell and Josiah Willard Gibbs
For sale as Licensed Images
Choose your image, Select your licence and Download the media
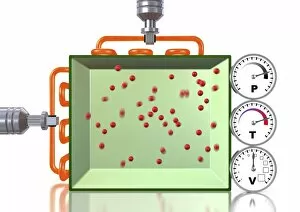
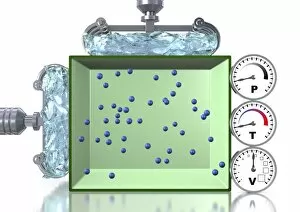
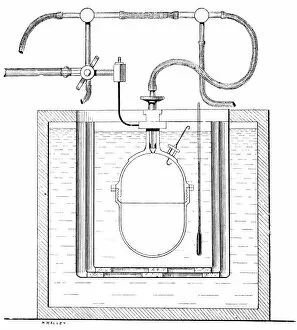
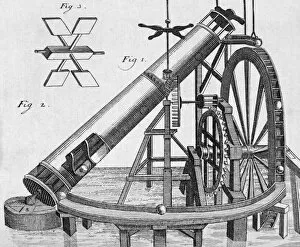
Thermodynamics, a fascinating branch of physics, owes its foundations to the brilliant minds of James Clerk Maxwell and Josiah Willard Gibbs. Scottish physicist James Clerk Maxwell is renowned for his groundbreaking work in electromagnetism, but he also made significant contributions to thermodynamics. One of his notable concepts was the "Maxwell's demon, " which challenged the second law of thermodynamics. Speaking of demons, let's not forget about Lord Kelvin and his compass. This Scottish mathematician and physicist played a crucial role in advancing our understanding of thermodynamics. In 1902, artist James Craig Annan captured Lord Kelvin with his trusty compass in an exquisite portrait. When discussing thermodynamics, we cannot overlook the three states of matter: ice, water, and steam. These states exemplify how energy can transform from one form to another while obeying fundamental laws like conservation. Another prominent figure in this field is William Thomson (also known as Lord Kelvin). His oil painting from 1886 showcases him as a distinguished scientist who made substantial contributions to many scientific disciplines. The concept of equilibrium is vital in thermodynamics. A captivating pen & ink drawing titled "Demonstration of the Third Equilibrium" depicts an experiment conducted around 1800 that explored this principle further. Benjamin Thompson deserves recognition too; he was an engraver whose work contributed significantly to early understandings of heat transfer and thermal conductivity. Fast forward to Professor Walther Nernst in 1928 – although unknown creator – Nernst developed important theories related to chemical reactions under specific temperature conditions called absolute zero or zero entropy. Lastly, Sir William Thomson (Lord Kelvin) graces us again with a lithograph from around 1890. This Irish physicist and engineer left an indelible mark on thermodynamic theory during the late nineteenth century.