Zinc Collection (#4)
"Unearthing the Power of Zinc: From Boom Towns to Fruit-Powered Clocks" Step back in time to Leadville, a Colorado boom town in the 1870s
For sale as Licensed Images
Choose your image, Select your licence and Download the media
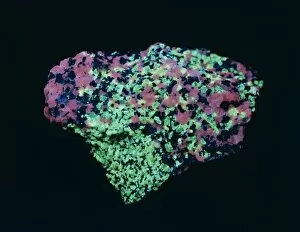
"Unearthing the Power of Zinc: From Boom Towns to Fruit-Powered Clocks" Step back in time to Leadville, a Colorado boom town in the 1870s, where zinc played a crucial role in shaping its history. Picture No. 11091681 captures the essence of this era, showcasing the resilience and determination of those who sought fortune amidst challenging conditions. But zinc's influence didn't stop there; it extended even to modern innovations like central heating for cars. Thanks to W H Robinson's ingenuity, vehicles became more comfortable and efficient with the help of this remarkable metal. Mining Franklinite, a rich source ore, was no easy task. Yet miners braved harsh conditions at Frongoch lead and zinc mine near Pontrhydygroes in Wales during the early 1900s. Their dedication ensured that this valuable resource could be extracted and utilized effectively. Across continents, Broken Hill (now Kabwe) witnessed another significant mining operation depicted in a diagram from Northern Rhodesia (Zambia). This lead and zinc mine served as an economic lifeline for many communities while contributing to global trade networks. The beauty found within these mines is awe-inspiring. Chalcopyrite with Quartz and Minor Sphalerite from the United Kingdom showcases nature's artistry at its finest. Similarly, Calcite from Ball Eye Mine in Cromford, Derbyshire exudes elegance while reminding us of zinc's geological significance. In Ashover, Derbyshire England Galena, Sphalerite Bitumen and Fluorite intertwine harmoniously creating stunning formations that captivate our imagination. Beyond industrial applications lies an unexpected connection between zinc and everyday life – a fruit-powered clock. Yes. The wonders never cease as we discover how this essential mineral can power time itself. Finally yet importantly - let us not forget about nurturing life on Earth.